Limiting & excess reactant example #1 Limiting reagents Limiting excess reactants reactant mass example reaction stoichiometry chemistry moles chapter much produced remains after given chemical hcl two solution
PPT - The Mathematics of Chemical Equations Chapter 11 PowerPoint
Calculating excess reactant uses and left over Limiting reactant and excess reactant How to find excess reactant left over : an excess reactant is the
Limiting reactant excess chemistry reagent chemical reaction science tyler dewitt introduction reactions equation help extra innov8tiv saved
Excess reactant limitingStoichiometry example: calculating the amount of excess reactant Excess reactant remainingChemistry limiting water reagents making reactant introductory chemical molecules use reactions atoms before many figure scenario run courses peoi chemintro.
Limiting excess reagents reactant reagent reactants reaction chemistry which formulas yieldLimiting reactant Limiting and excess reagentsExcess reactant amount stoichiometry calculating example.

Reactant limiting excess stoichiometry moles
Introduction to limiting reactant and excess reactantReactant limiting excess powerpoint reactants ppt presentation Limiting reactant excess yield socraticExcess reactant equations mathematics chemical chapter ppt powerpoint presentation.
Limiting excess reactant reactants stoichiometry nacl ppt powerpoint presentation slideserveExcess reactant remaining Excess reactant limiting exampleExcess reactant amount reaction limiting reacted.
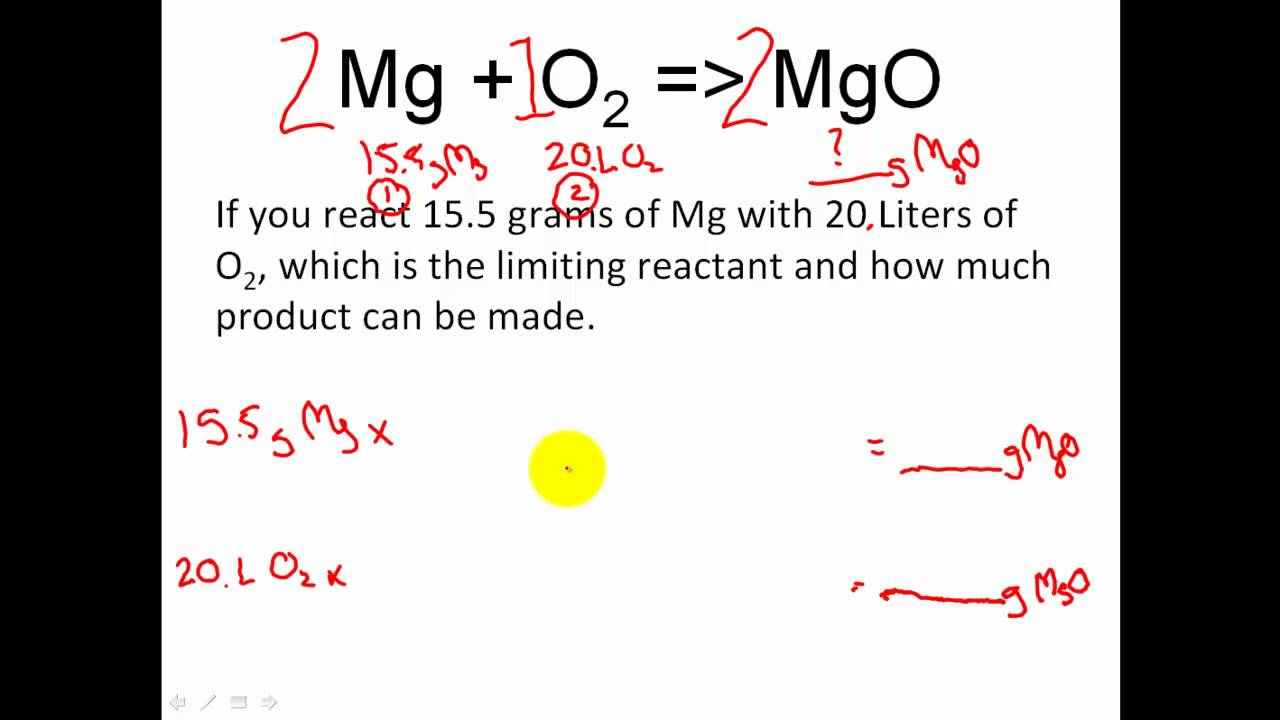
Excess reactant
What is the difference between a limiting reactant and an excess .
.


Limiting Reactant - Solution Stoichiometry

Limiting Reagents

Stoichiometry Example: Calculating the amount of excess reactant - YouTube

PPT - Stoichiometry PowerPoint Presentation, free download - ID:1674781

PPT - The Mathematics of Chemical Equations Chapter 11 PowerPoint

PPT - Limiting reactant PowerPoint Presentation, free download - ID:2568932

Introduction to Limiting Reactant and Excess Reactant - YouTube

Limiting reactant and excess reactant

Excess Reactant Remaining - YouTube
